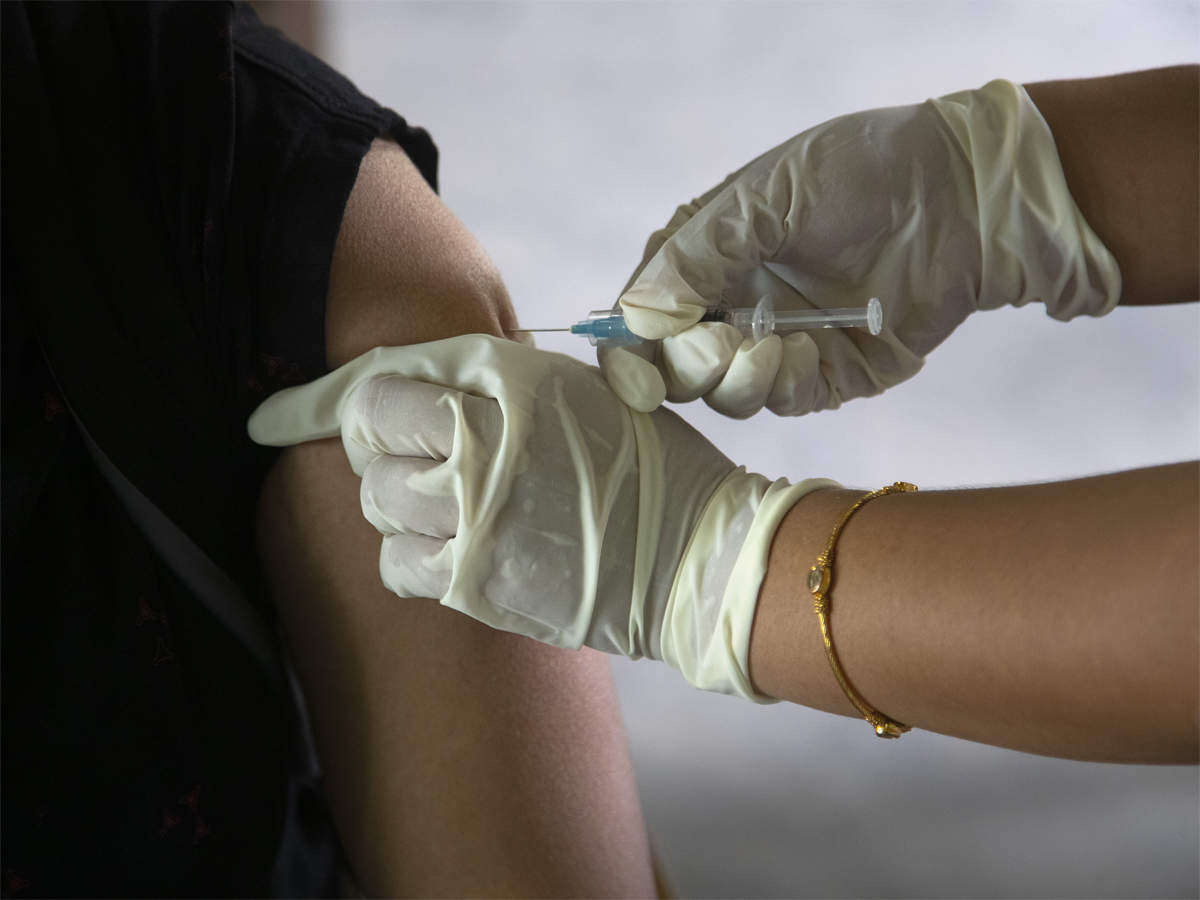
 The Subject Expert Committee on COVID-19 of the Central Drugs Standard Control Organization deliberated upon Hyderabad-based Bharat Biotech's application seeking permission to conduct phase II/III clinical trials to evaluate the safety, reactogenicity and immunogenicity of Covaxin jabs in children aged 2 to 18 years.
The Subject Expert Committee on COVID-19 of the Central Drugs Standard Control Organization deliberated upon Hyderabad-based Bharat Biotech's application seeking permission to conduct phase II/III clinical trials to evaluate the safety, reactogenicity and immunogenicity of Covaxin jabs in children aged 2 to 18 years.from Healthcare/Biotech-Industry-Economic Times https://ift.tt/3o7KLWx
via IFTTT






0 comments:
Post a Comment